Lanthanide-Porphyrin Hybrids: from Layered Structures to MOFs
J. Demel, P. Kubát, F. Millange, J. Marrot, I. Císařová, K. Lang
Inorg. Chem. 2013, 52, 2779-2786
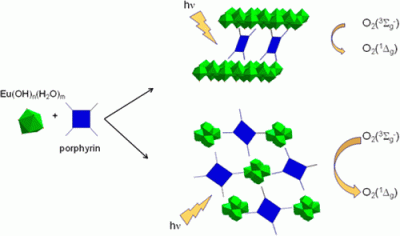
Abstract: Rare-earth layered hydroxides with intercalated tetrasulfonated porphyrins and corresponding to the chemical formula Ln2(OH)4.7(Por)0.33·2H2O (Ln = Eu3+, Tb3+; Por = 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin (TPPS) and PdTPPS) have been prepared to investigate their photophysical properties. A slight variation of the synthetic procedure led to the metal–organic framework (MOF) assembled from a distorted octahedral oxometalate clusters [Eu6(μ6-O)(μ3-OH)8(H2O)14]8+. These secondary building units (SBUs) are linked together by six distorted porphyrin units. During activation, the original SBU loses not only water molecules from the coordination sphere but also the central μ6-O atom. The loss of the central atom results in the distortion of the octahedral [Eu6(μ6-O)(μ3-OH)8(H2O)14]8+ SBU into a trigonal antiprismatic [Eu6(μ3-OH)8(H2O)2]10+ SBU with two μ3-OH groups nearly in plane with the europium atoms and the reduction of pores to approximately 2 × 3 Å. As a result, the MOF has no accessible porosity. This transformation was thoroughly characterized by means of single-crystal X-ray crystallographic analysis of both phases. Solid-state photophysical investigations suggest that the MOF material is fluorescent; however, in contrast to the prepared layered hydroxides, the as-prepared MOF is an effective sensitizer of singlet oxygen, O2(1Δg), with a relatively long lifetime of 23 ± 1 μs. The transition is also accompanied by variation in photophysical properties of the coordinated TPPS. The alteration of the fluorescence properties and of the O2(1Δg) lifetime presents an opportunity for preparation of MOFs with oxygen-sensing ability or with oxidation potential toward organic molecules by O2(1Δg).