Light-emitting materials based on icosahedral boron clusters. Structure, photophysics and applications in bioimaging
Rosario Núñez,1* Mahdi Chaari,1 Justo Cabrera-González,1 Nerea Gaztelumendi,2 Carme Nogués,2 Clara Viñas1 and Francesc Teixidor 1
1Institut de Ciència de Materials de Barcelona (ICMAB-CSIC), Campus de la UAB, E-08193, Bellaterra (Barcelona), Spain. rosario@icmab.es
2Departament de Biologia Cel·lular, Fisiologia i Immunologia. Universitat Autònoma de Barcelona, Campus de la UAB, E-08193 Bellaterra (Barcelona), Spain.
Icosahedral boron clusters are very robust compounds characterized by a three-dimensional (3D) s-delocalization,1 high thermal and chemical stability, hydrophobicity and low toxicity in biological systems.2 Due to their unique structural and electronic properties, carborane clusters are excellent entities when applying them to tailor the fluorescence emission of any fluorophore group.3
We have designed efficient light-emitting icosahedral boron cluster-containing molecules, whose emission in solution depends critically on the nature of the cluster and substituents. It was observed that molecules showing an almost lack of fluorescence in solution were able to exhibit intense aggregation induced emission (AIE) in solid state. In this way, and depending on the application of choice, we are capable of preparing highly fluorescent materials in both states.4a
We have developed boron cluster-based conjugates as fluorescent probes for in vitro studies, by linking different neutral or anionic boron clusters (carborane C2B10H12, metallacarboranes [M(C2B9H11)2]– (M = Co, Fe), and closo-dodecaborate [B12H12]2- moieties) to anthracene and BODIPY derivatives. 4b,5 Their exceptional cellular uptake and intracellular boron release, together with their fluorescence properties, biocompatibility and low toxicity make these compounds good candidates for cell tracking and boron delivery systems.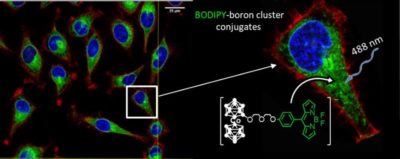 Figure 1. Confocal microscopy images of HeLa cells incubated with BODIPY-Cosane conjugates emitting green fluorescence.
Figure 1. Confocal microscopy images of HeLa cells incubated with BODIPY-Cosane conjugates emitting green fluorescence.
- J. Poater, M. Solà, C. Viñas and F. Teixidor, Angew. Chem. Int. Ed., 2014, 53, 12191-12195.
- a) Viñas, C. Future Med. Chem. 2013, 5, 617-619. b) Hey-Hawkins E., Viñas, C. En Boron-Based Compounds: Potential and Emerging Applications in Medicine, Wiley, 2018.3.
- R. Núñez, M. Tarrés, A. Ferrer-Ugalde, F. Fabritzi de Biani, F. Teixidor, Chem. Rev., 2016. 116(23), 14307-14378.
- a) M. Chaari, Z. Kelemen, J. G. Planas, F. Teixidor, D. Choquesillo-Lazarte, A. Ben Salah, C. Viñas, R. Núñez, J. Mater. Chem. C, 2018, 6, 11336. b) M. Chaari, Z. Kelemen, D. Choquesillo-Lazarte, N. Gaztelumendi, F. Teixidor, C. Viñas, C. Nogués, R. Núñez, Biomater. Sci., 2019, DOI: 10.1039/c9bm00903e.
- a) M. Chaari, N. Gaztelumendi, J. Cabrera-González, P. Peixoto-Moledo, C. Viñas, E. Xochitiotzi-Flores, N. Farfán, A. Ben Salah, C. Nogues, R. Núñez, Bioconjugate Chem., 2018, 29, 1763-1773. c) C. Bellomo, M. Chaari, J. Cabrera-González, M. Blangetti, C. Lombardi, A. Deagostino, C. Viñas, C.; N. Gaztelumendi, C. Nogués, R. Núñez, C. Prandi, Chem. Eur. J., 2018, 24, 15622.
