Luminescent cationic group 4 metallocene complexes with chelating ligands
David Dunlop,a Béla Urbán,a Róbert Gyepes,a,b Pavel Kubát,a Kamil Lang,c Michal Horáček,a Ludmila Ši
mková,a and Martin Lamača
a J. Heyrovský Institute of Physical Chemistry, Academy of Sciences of the Czech Republic, v.v.i., Dolejškova 2155/3, 18223 Praha 8, Czech Republic;
b Department of Inorganic Chemistry, Faculty of Science, Charles University in Prague, Hlavova 2030, 12840 Praha 2, Czech Republic;
c Institute of Inorganic Chemistry, Academy of Sciences of the Czech Republic, v.v.i., 25068 Řež, Czech Republic; E-mail address: martin.lamac@jh-inst.cas.cz
Photoluminescent metal complexes have a widespread application potential in various fields such as photocatalysis, photovoltaics, light-emitting devices, molecular sensors, bioimaging, etc., but among them the complexes of electron-deficient metals are still rather underexplo
red. Such compounds form potentially luminescent excited states by ligand-to-metal charge transfer (LMCT)
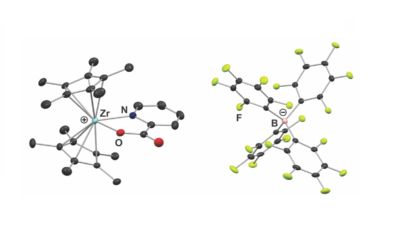
unlike those based on late transition metals or lanthanides. We have recently observed luminescent behavior of Zr and Hf metallocene type complexes with intramolecularly tethered iminium moiety that were obtained as cationic species by protonation of the ketimide group in parent neutral complexes.1,2 As an extension, we have prepared a new series of related easily accessible cationic metallocenes with N,O-chelating heteroaromatic ligands that also exhibit photoluminescence (emission maxima 520-575 nm) both in the solid state and in solution at ambient temperature with relatively high quantum yields and luminescence lifetimes up to tens of µs.
References
- (a) Večeřa, M.; Varga, V.; Císařová, I.; Pinkas, J.; Kucharczyk, P.; Sedlařík, V.; Lamač, M. Organometallics 2016, 35, 785; (b) Varga, V; Večeřa, M.; Gyepes, R.; Pinkas, J.; Horáček, M.; Merna, J.; Lamač, M. ChemCatChem 2017, 9, 3160.
- Dunlop, D.; Večeřa, M.; Gyepes, R.; Kubát, P.; Pinkas, J.; Horáček, M.; Lamač, M.: manuscript in preparation.